In chemistry, a metal (Greek: Metallo, Μέταλλο) is a chemical element whose atoms readily lose electrons to form positive ions (cations), and form metallic bonds between other metal atoms and ionic bonds between nonmetal atoms.[1]
Definition
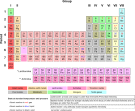
Metals are sometimes described as a lattice of positive ions surrounded by a cloud of delocalized electrons. They are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. On the periodic table, a diagonal line drawn from boron (B) to polonium (Po) separates the metals from the nonmetals. Most elements on this line are metalloids, sometimes called semi-metals; elements to the lower left are metals; elements to the upper right are nonmetals (see the periodic table showing the metals).
An alternative definition[citation needed][unreliable source?] of metals is that they have overlapping conduction bands and valence bands in their electronic structure[2]. This definition opens up the category for metallic polymers and other organic metals, which have been made by researchers and employed in high-tech devices. These synthetic materials often have the characteristic silvery-grey reflectiveness (luster) of elemental metals.
Chemical properties
Metals are usually inclined to form cations through electron loss,[1] reacting with oxygen in the air to form oxides over changing timescales (iron rusts over years, while potassium burns in seconds). Examples:
- 4Na + O2 → 2Na2O (sodium oxide)
- 2Ca + O2 → 2CaO (calcium oxide)
- 4Al + 3O2 → 2Al2O3 (aluminium oxide)
The transition metals (such as iron, copper, zinc, and nickel) take much longer to oxidize. Others, like palladium, platinum and gold, do not react with the atmosphere at all. Some metals form a barrier layer of oxide on their surface which cannot be penetrated by further oxygen molecules and thus retain their shiny appearance and good conductivity for many decades (like aluminium, some steels, and titanium). The oxides of metals are basic (as opposed to those of nonmetals, which are acidic), although this may be considered a rule of thumb, rather than a fact.
Painting, anodising or plating metals are good ways to prevent their corrosion. However, a more reactive metal in the electrochemical series must be chosen for coating, especially when chipping of the coating is expected. Water and the two metals form an electrochemical cell, and if the coating is less reactive than the coatee, the coating actually promotes corrosion.
Physical properties

Metals in general have superior electric and thermal conductivity, high luster and density, and the ability to be deformed under stress without cleaving.[1] While there are several metals that have low density, hardness, and melting points, these (the alkali and alkaline earth metals) are extremely reactive, and are rarely encountered in their elemental, metallic form.[1]
Density
The majority of metals have higher densities than the majority of nonmetals.[1] Nonetheless, there is wide variation in the densities of metals; lithium is the least dense solid element and osmium is the densest. The metals of groups I A and II A are referred to as the light metals because they are exceptions to this generalization[1]. The high density of most metals is due to the tightly-packed crystal lattice of the metallic structure. The strength of metallic bonds for different metals reaches a maximum around the center of the transition series, as those elements have large amounts of delocalized electrons in a metallic bond. However, other factors (such as atomic radius, nuclear charge, number of bonding orbitals, overlap of orbital energies, and crystal form) are involved as well.[1]
Malleability
The nondirectional nature of metallic bonding is thought to be the primary reason for the malleability of metal. Planes of atoms in a metal are able to slide across one another under stress, accounting for the ability of a crystal to deform without shattering.

When the planes of an ionic bond are slid past one another, the resultant change in location shifts ions of the same charge into close proximity, resulting in the cleavage of the crystal. Covalently bonded crystals can only be deformed by breaking the bonds between atoms, thereby resulting in fragmentation of the crystal.
Conductivity
The electrical and thermal conductivity of metals originate from the fact that in the metallic bond, the outer electrons of the metal atoms form a gas of nearly free electrons, moving as an electron gas in a background of positive charge formed by the ion cores. Good mathematical predictions for electrical conductivity, as well as the electrons' contribution to the heat capacity and heat conductivity of metals can be calculated from the free electron model, which does not take the detailed structure of the ion lattice into account.
Electric charge
When considering the exact band structure and binding energy of a metal, it is necessary to take into account the positive potential caused by the specific arrangement of the ion cores - which is periodic in crystals. The most important consequence of the periodic potential is the formation of a small band gap at the boundary of the brillouin zone. Mathematically, the potential of the ion cores is treated in the nearly-free electron model.
Alloys
An alloy is a mixture of two or more elements in solid solution in which the major component is a metal. Most pure metals are either too soft, brittle or chemically reactive for practical use. Combining different ratios of metals as alloys modifies the properties of pure metals to produce desirable characteristics. The aim of making alloys is generally to make them less brittle, harder, resistant to corrosion, or have a more desirable color and luster. Examples of alloys are steel (iron and carbon), brass (copper and zinc), bronze (copper and tin), and duralumin (aluminium and copper). Alloys specially designed for highly demanding applications, such as jet engines, may contain more than ten elements.
Categories
Base metal
In chemistry, the term 'base metal' is used informally to refer to a metal that oxidizes or corrodes relatively easily, and reacts variably with dilute hydrochloric acid (HCl) to form hydrogen. Examples include iron, nickel, lead and zinc. Copper is considered a base metal as it oxidizes relatively easily, although it does not react with HCl. It is commonly used in opposition to noble metal.
In alchemy, a base metal was a common and inexpensive metal, as opposed to precious metals, mainly gold and silver. A longtime goal of the alchemists was the transmutation of base metals into precious metals.
In numismatics, coins used to derive their value primarily from the precious metal content. Most modern currencies are fiat currency, allowing the coins to be made of base metal.
Ferrous metal
The term "ferrous" is derived from the latin word meaning "containing iron". This can include pure iron, such as wrought iron, or an alloy such as steel. Ferrous metals are often magnetic, but not exclusively.
Noble metal
Noble metals are metals that are resistant to corrosion or oxidation, unlike most base metals. They tend to be precious metals, often due to perceived rarity. Examples include tantalum, gold, platinum, and rhodium.
Precious metal

A precious metal is a rare metallic chemical element of high economic value.
Chemically, the precious metals are less reactive than most elements, have high luster and high electrical conductivity. Historically, precious metals were important as currency, but are now regarded mainly as investment and industrial commodities. Gold, silver, platinum and palladium each have an ISO 4217 currency code. The best-known precious metals are gold and silver. While both have industrial uses, they are better known for their uses in art, jewelry, and coinage. Other precious metals include the platinum group metals: ruthenium, rhodium, palladium, osmium, iridium, and platinum, of which platinum is the most widely traded. Plutonium and uranium could also be considered precious metals.
The demand for precious metals is driven not only by their practical use, but also by their role as investments and a store of value. Palladium was, as of summer 2006, valued at a little under half the price of gold, and platinum at around twice that of gold. Silver is substantially less expensive than these metals, but is often traditionally considered a precious metal for its role in coinage and jewelry.
Tidak ada komentar:
Posting Komentar